There are diseases whose pathogenesis has been known for decades. The actual cause, however, remains a secret despite intensive research. For decades, it has been known that deposits of immune complexes are formed along the basal membrane of the glomerulus in membranous nephropathy (previously known as membranous glomerulonephritits), causing tissue inflammation and impairment of kidney filtration. But the antigen which is targeted by autoantibodies in human membranous nephropathy could not be identified for many years.
But finally, since 2009, the secret has been disclosed: It is the phospholipase A2 receptor 1 (PLA2R). Approximately 70% of patients suffering from primary membranous nephropathy reveal autoantibodies against PLA2R. This finding served as foundation for the development of novel diagnostic test systems by EUROIMMUN.
Important information about PLA2R and membranous nephropathy
- Membranous nephropathy is an inflammatory disease of the glomerulus and leads to proteinuria, impaired fat metabolism and potential renal failure; proteinuria functions as a clinical marker for disease activity
- Primary membranous nephropathy is a kidney-specific autoimmune disease; secondary membranous nephropathy is caused by another underlying disease
- Anti-PLA2R autoantibodies are the only biomarker for primary membranous nephropathy known so far
- Detection of anti-PLA2R autoantibodies may allow differentiation between primary and secondary membranous nephropathy; this is crucial for an appropriate treatment
- Anti-PLA2R titer allows predicitions about therapy success and risk of membranous nephropathy relapse after kidney transplantation
Diagnosing membranous nephropathy
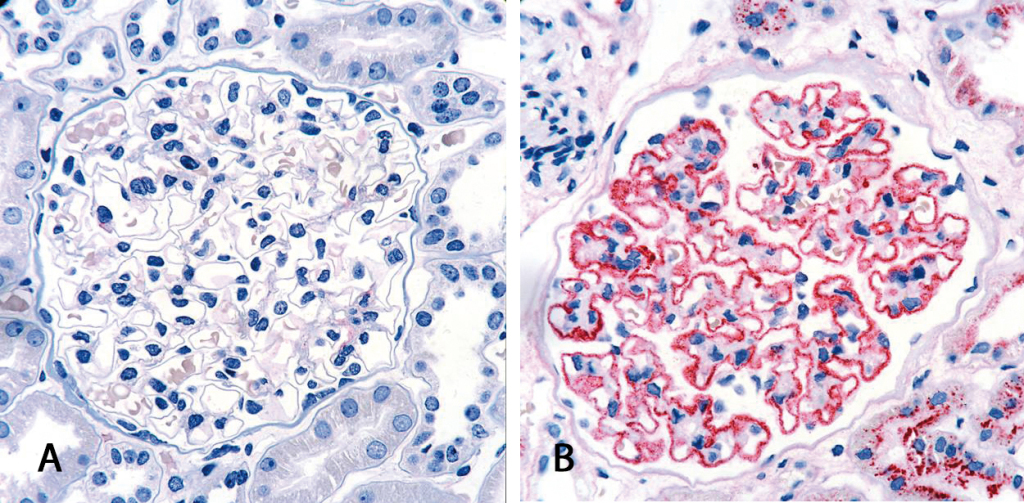
Diagnosis of membranous nephropathy is commonly based on kidney biopsy. The tissue is analyzed histologically and in the case of a positive result, immune deposits can be detected along the basal membrane of the glomerulus. This surgical intervention represents a mentally and physically stressful situation for each patient. The discovery of anti-PLA2R antibodies as specific biomarker for primary membranous nephropathy is of major relevance for future diagnostics as it allows a non-invasive form of diagnostics with the help of simple serological test systems.
Anti-PLA2R tests facilitate diagnostics
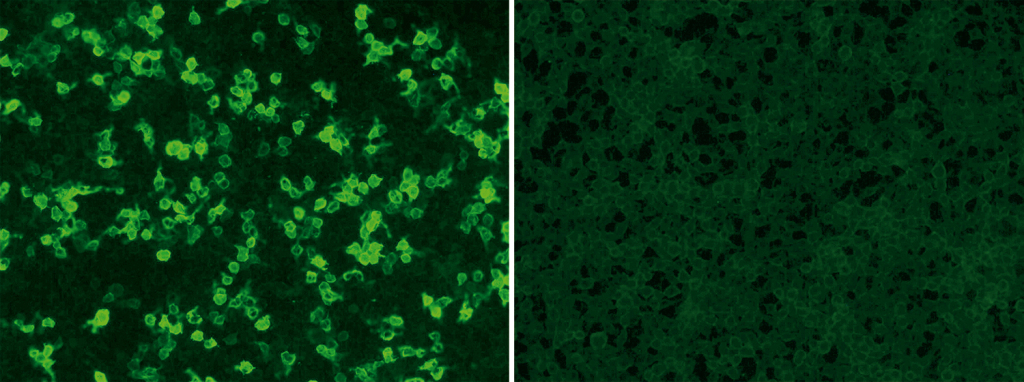
EUROIMMUN offers standardized and commercial tests for qualitative and quantitative detection of circulating anti-PLA2R autoantibodies: the indirect immunofluorescence assay uses recombinant cells as standard substrate (see post image: positive reaction of patient sample with tranfected cells [left], controll [right]; Anti-PLA2R RC-IFA, order number: FA 1254-###-50) while the purified receptor serves as antigen for coating the plates of the enzyme-linked-immunosorbent-assay (Anti-PLA2R ELISA (IgG), order number: EA 1254-9601 G). Both tests require only small volumes of blood. The blood withdrawal is considerably less burdensome for the patient compared to a biopsy. The tests are certificated with the CE Label and have been released by the US Food and Drug Administration, recently.
The Anti-PLA2R immunofluorescence assay
The Anti-PLA2R RC-IFA (IgG) is suitable for fast and easy screening of samples from patients with suspected membranous nephropathy. Autoantibodies against PLA2R can be qualitatively and semi-quantitatively detected by this reliable test system.
In May 2014, the Anti-PLA2R RC-IFA (IgG) was approved for sale in the USA by FDA. Its release was a particular success because it represents a de-novo classification process of a novel medical device. The indirect immunofluorescence assay was explicitly praised as a helpful screening test. It would allow timely diagnosis and early treatment of affected patients, stated Alberto Gutierrez, the director of the Office of In Vitro Diagnostics at the FDA in its press announcement.
The Anti-PLA2R ELISA
The Anti-PLA2R ELISA (IgG) is ideally suitable for screening of large sample collectives. Additionally it enables the quantitative determination of the anti-PLA2R antibody titer.
Similar to proteinuria, the anti-PLA2R titer correlates with disease activity. However, the titer responds more sensitively to alterations in disease course or to immunosuppressive therapy. This is why the anti-PLA2R titer is suitable for disease and therapy monitoring. Furthermore, the anti-PLA2R titer has a predictive value since high anti-PLA2R levels may indicate that a more intense and long-term treatment is needed to achieve remission of symptoms. High antibody titers are moreover considered a significant risk factor of membranous nephropathy relapse after kidney transplantation.
This test as well was approved by the FDA in July 2014 and is now sold in the USA.