Sometimes, the answer yes or no or the result positive or negative is not sufficient. For example with human papillomaviruses (HPV) infections, it is important for the physician and the patient to receive more information from a diagnostic test, because in these cases positive can have more than just one meaning.
Important information about HPV
- HPV is the cause of cervical cancer
- Over 100 different HPV variants (subtypes) exist; 30 HPV subtypes may infect the anogenital tissue
- 12 low-risk HPV subtypes and 18 high-risk HPV subtypes are differentiated; only high-risk HPV subtypes can cause malignant alterations of the endometrium
- HPV infections belong to the most abundant sexually transmitted diseases; over 80% of humans worldwide will catch the virus during their lives, but most of them will not develop any symptoms
- In Germany, routine cervical cancer screening is based on the cytological pap test which allows recognition of cellular alterations within the endometrium; its visual interpretation is considered to be error-prone
- Molecular HPV tests enable earlier recognition of infection and prevention of tumour formation
Molecular HPV test
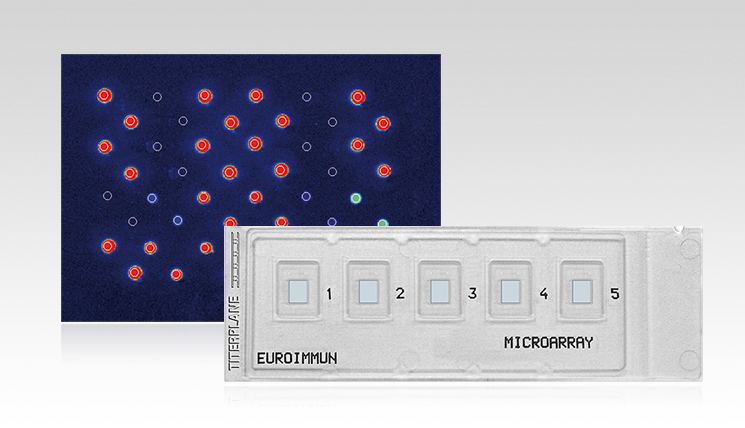
The EUROArray HPV is a molecular microarray to detect and identify the 30 clinically relevant HPV subtypes which are linked to the transformation of cells in anogenital tissues (order number: MN 2540-####). DNA isolated from epithelial cells from cervical smears serves as sample material. A PCR is performed to specifically amplify viral DNA and label it with a fluorescence dye. With the help of the microarray, the viral DNA then can be detected.

Microarrays by EUROIMMUN are provided on specific microscope slides in the BIOCHIP format. One BIOCHIP harbors 72 spots of specific single-stranded DNA probes. Every spot corresponds to one of the 30 HPV subtypes or serves as internal control during the evaluation process. This evaluation is performed automatically using the microarray scanner and the EUROArrayScan software (order number: YG 0601-0101).
The major advantage: performance of the EUROArray HPV does not require molecular biology expertise. Every step is easy to do and interpretation and storage of data occurs automatically.
Speaking of the evaluation …
Positive – and what else?
The HPV test is positive as soon as the EUROArray detects one HPV subtype. But the HPV test provides further important information. The microarray allows the exact identification of the HPV subtype the patient has caught, if it belongs to the 30 relevant HPV subtypes – 18 high-risk HPV and 12 low-risk HPV subtypes. This subtyping enables the physician to estimate and predict the individual risk of cervical cancer development for each woman. An infection with a low-risk HPV type is no reason to worry. Low-risk HPV types do not cause cancer, but maybe genital warts, according to the current state of scientific knowledge. Only high-risk HPV types are carcinogenetic. HPV types 16 and 18 can be found in 70% of cervical cancers. Especially persistent and multiple infections with several high-risk HPV types increase the cervical cancer risk. With the EUROArray HPV result for a patient, the physician is able to evaluate her individual risk of developing cervical cancer before any obvious cytological alterations occur. In most cases cancer can then be prevented by timely recognition, monitoring and adequate treatment of a HPV infection.